Clinical Trials at
Houston Mind & Brain
Join a Research Study!
Clinical trials help advance medical knowledge and improve care for patients by evaluating the safety and effectiveness of experimental therapies
Clinical research at HMB offers:
Access to the newest treatments available
Compensation for your time
Education about your mental illness
Opportunity to advance psychiatric knowledge and clinical care
For more information on our specific studies, please fill out this form:
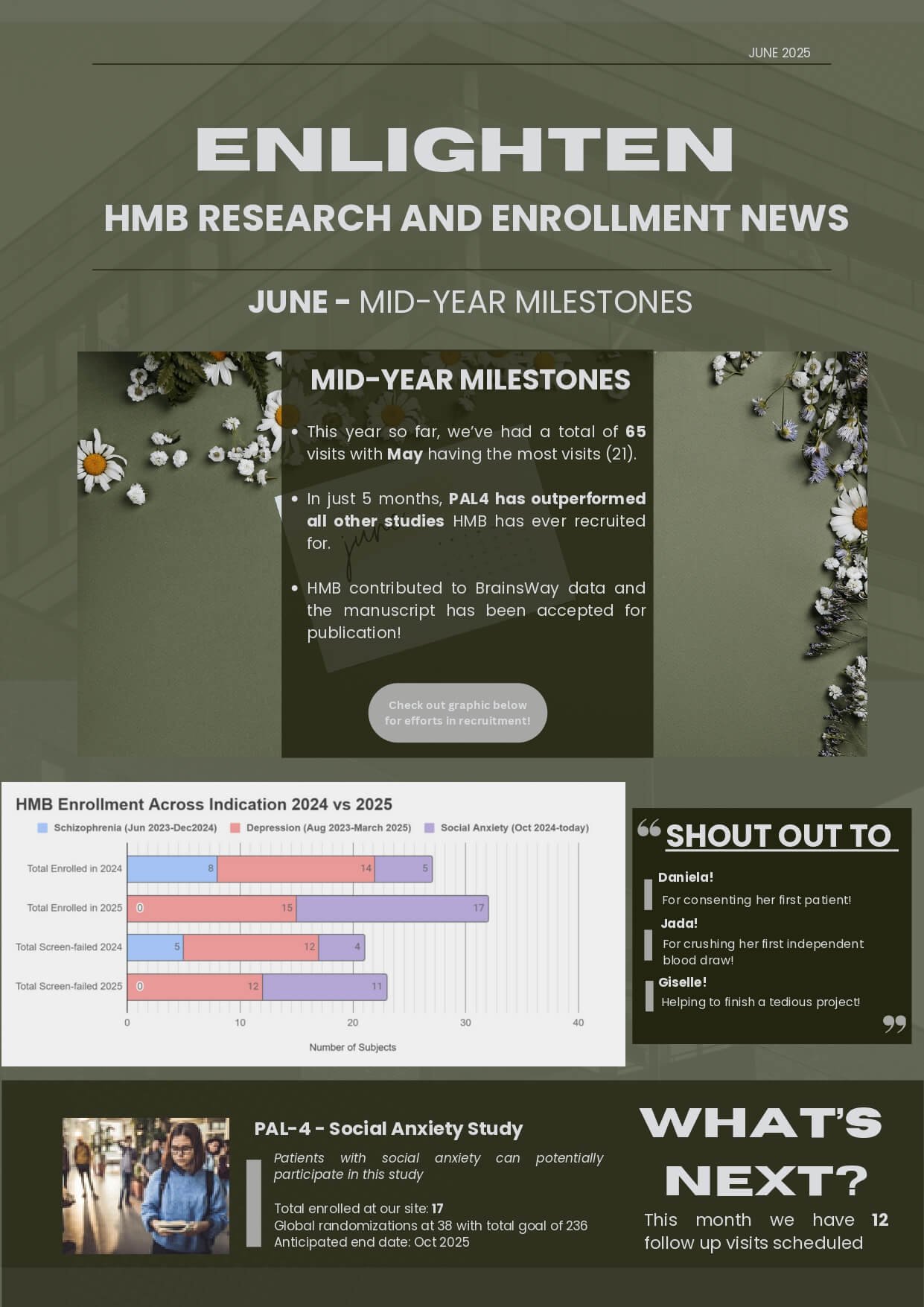
We are currently enrolling for the following studies:
A Phase 2, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-arm Trial to Assess the Efficacy, Safety, and Tolerability of Centanafadine Extended-release Capsules as Monotherapy or as Adjunct to SSRI in Adult Subjects with Major Depressive Disorder.
Are you experiencing a low or depressed mood and a decreased interest in pleasurable activities?
Do you have feelings of guilt or worthlessness, lack of energy, or poor concentration?
This research study is being conducted to assess the effectiveness, safety, and tolerability of centanafadine once-daily extended-release capsules for the possible treatment of adult participants with MDD who have reported inadequate response to at least 1 but no more than 3 treatments for depression in their current major depressive episode (MDE). This research study will test the hypothesis that centanafadine as monotherapy or in addition to SSRI (escitalopram) will lead to greater overall effectiveness in the possible treatment of MDD when compared to escitalopram alone or to placebo.

PAL-4 / A US, Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Clinical Trial of Fasedienol Nasal Spray for the Acute Treatment of Anxiety Induced by a Public Speaking Challenge in Adult Subjects with Social Anxiety Disorder, with an Open-Label Extension (PALISADE-4)
This study is investigating whether a nasal spray can help with acute symptoms of social anxiety. There is a double blind, randomized phase and an optional open label extension where you would receive the study drug for up to 1 year.
Social Anxiety Disorder (SAD) can involve intense feelings of nervousness and anxiety when faced with a situation involving social interaction, like attending a party or speaking up at a meeting. Those with SAD frequently forgo both professional and social relationships, missing out on activities that are important to them. They fear being judged or scrutinized by others. They may understand that their fears are irrational or unreasonable, but feel powerless to overcome them. SAD goes beyond being “shy” or “introverted”.
Approximately 15 million U.S. adults have SAD and are under-treated. SAD can have physical symptoms that manifest in many ways, which may include blushing, nausea, sweating, shaking/trembling, dizziness, rapid heart rate, SAD can have psychological symptoms that may include intense worry about or avoidance of social situations, deep concern about what others think of you, and feeling embarrassed, humiliated or ashamed.
To enroll in this clinical trial, you must be:
- Diagnosed (or get diagnosed) with Social Anxiety Disorder
- Between the ages of 18 & 65
- Willing to visit our clinic for study related appointments
- Otherwise in good, stable health physically and mentally
This study is investigating whether a nasal spray can help with acute symptoms of social anxiety. There is a double blind, randomized phase and an optional open label extension where you would receive the study drug for up to 1 year.